In case of mild nervous tension, mental stress, sleep troubles, it is recommended that the medical treatment starts with the administration of herbal materials with a calming effect. Conventional pharmacological solutions used in the therapy of anxiety disorders do not always manage to remove all undesirable symptoms (Kinrys et al 2009). Moreover, like hypnotic medications, (Yarnell 2015) they may cause a number of burdensome side effects (Rudolph and Knoflach 2011).
Because of that, there is a growing interest in options of alleviating anxiety (Kinrys et al. 2009) and sleeplessness (Yarnell 2015) with vegetal solutions alternative and/or complementary to conventional means (Kinrys et al. 2009, Yarnell 2015).
For increased anxiety, emotional tension and/or sleeplessness the traditional medicine has recommended for long the extracts from the valerian root, Melissa herbaceous plant, passion flower or hop flowers (cones). This has been reflected in the relevant Community monographs, developed by the European Medicines Agency (EMA) [1,2,3,4]; they confirm that the said extracts may be used to reduce mental stress symptoms, mild nervous tension and difficulties falling asleep. Moreover, products obtained from valerian, Melissa, passion flower or hop are also commonly recommended for women who struggle with the burdensome perimenopausal symptoms.
Active substances with anxiolytic effects present in the valerian root extract include valerenic acid (Benke et al. 2009, Khom et al. 2007) and valerenol (Benke et al. 2009), which are β2/3-selective modulators of GABAA receptors (Benke et al. 2009, Khom et al. 2007). In low and moderate concentrations, the valerian extract also stimulates glutamate decarboxylase (GAD) – an enzyme acting as catalyst in GABA biosynthesis (Awad et al 2007). Important note: do not confuse valerenic acid with valeric acid which has no sedative properties.
In in vitro conditions Melissa leaf extract (Yoo et al. 2011) and rosmarinic acid (Awad et al. 2009) inhibits the activity of 4-aminobutyrate aminotrasferase (GABA-T), an enzyme responsible for the degradation of gamma-hydroxybutric acid (GABA) (Awad et al. 2007).
In turn, the anxiolytic action of passion flower extract may be attributed to its flavonoids (including vitexin) (Grundmann et al 2008) which bind with GABAA and GABAB receptors (Appel et al. 2011)
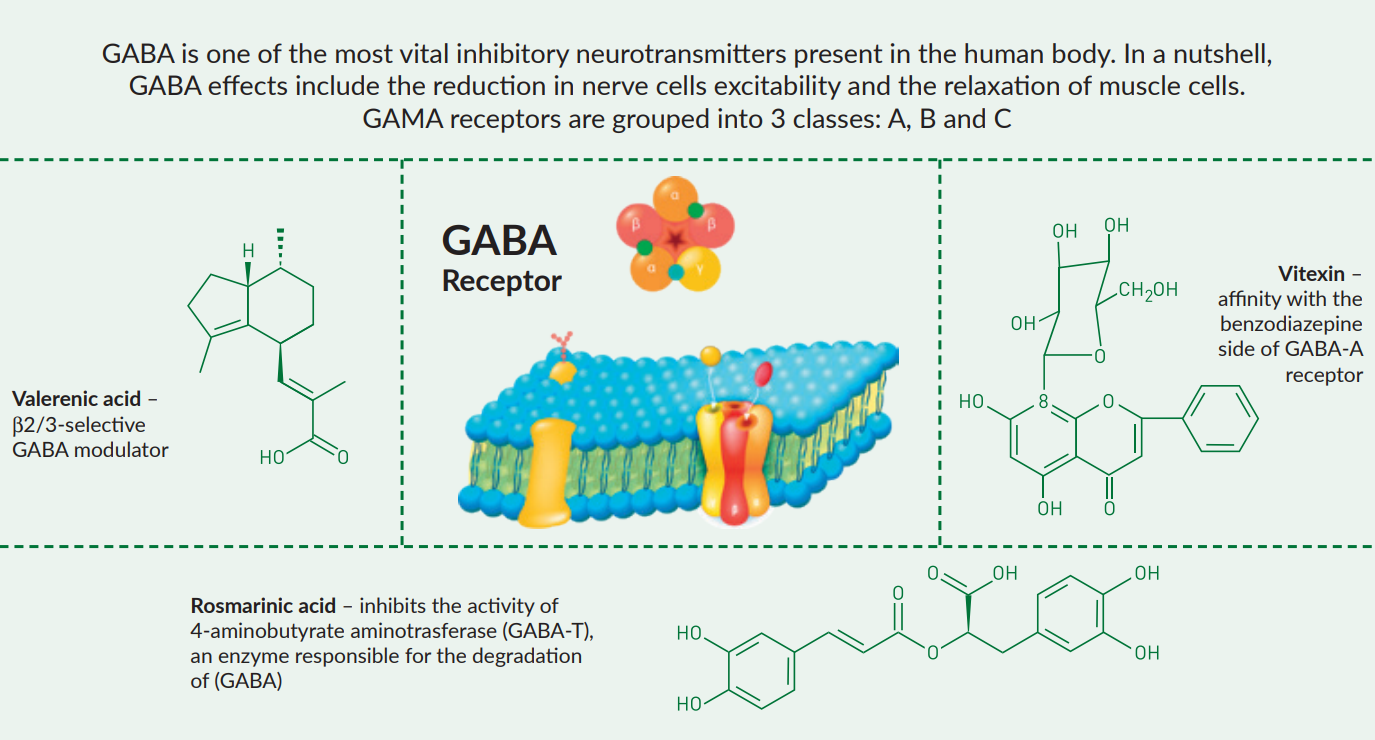
Even if anxiolytic and calming action of plant extracts may be the result of the aforesaid and broadly understood interactions with the GABAergic system (Awad et al. 2007), the comprehensive composition and awealth of active substancespresent in every of the said raw m aterials will naturally trigger other neuropharmacological mechanisms which are effective in anxiety disorders and in difficulties falling asleep. Valerenic acid, as one of the active substances present in the valerian extract, may also reduce the response to physical and psychological stressors by decreasing serotonin (5-HT / 5-HIAA¹) and neoadrenalin (NE/ MHPG-SO4²) turnovers in the hippocampus and in the amygdala (Yung et al. 2015). Finally, valerenic acid is also a partial agonist of 5-HT5a serotonin receptors (Dietz et al. 2005) which, as claimed by researchers, may be involved in the regulation of circadian rhythms (Duncan et al. 2000). Another study has demonstrated the ability of dry hop extract – with no bitter acids – to attach to the 5-HT6 serotonergic receptors and ML1 melatonergic receptors (Abourashed et al. 2004). The polyvalent action (Williamson et al. 2001) of formulations whichcombine the aforesaid plant extracts is evidenced by a number of scientific experiments, including observational studies conducted with the use of CALMOMIX® composition.
In 2020, GREENVIT conducted its own randomized study of the effects of CALMOMIX® complex on psychological well-being of patients in terms of sleep quality, ease of falling asleep, relief of nervous tension and irritability.
The double-blind study was conducted under the supervision of two investigators – psychiatric physicians on a group of 30 subjects. The volunteers were randomly divided into two groups. The first group took 2 capsules of placebo every evening, the second group took 2 capsules of the CALMOMIX® complex. The placebo and CALMOMIX® capsules looked identical. It was impossible to decode them. The application regime was maintained for 21 days. The average age in the placebo group was 43.5, in the CALMOMIX® group 38 years. Gender distributions in the CALMOMIX® and placebo groups were the same. There were no differences in gender distribution between groups. There was no relationship between the researcher assigned and the type of substance the participant received. The study was completed by 28 people (n=28). As an inclusion criterion, participants completed the DASS-21* self-report questionnaire prior to administration of the active substance and placebo. After the study was completed,
its participants completed the Visual Analogue Test (VAS). The test measured study participants’ perceptions in four areas: sleep quality, ease of falling asleep, nervous tension and irritability. In each of these four areas, the participant could select one of five responses. VAS was structured so that successive responses from one to five indicated increasing improvement of well-being in a given area. The study was structured so that an improvement was deemed to have occurred if, at the end of the study, the patient’s condition was better than before supplementation in at least one of the three areas examined.
Statistical analysis performed after collection of all the data of the 21-day study confirmed clear advantage of the CALMOMIX® complex over placebo in terms of clinical benefits: better ease of falling asleep, better sleep quality, reduced nervous tension.
No advantage of CALMOMIX® complex over placebo was found for irritability.
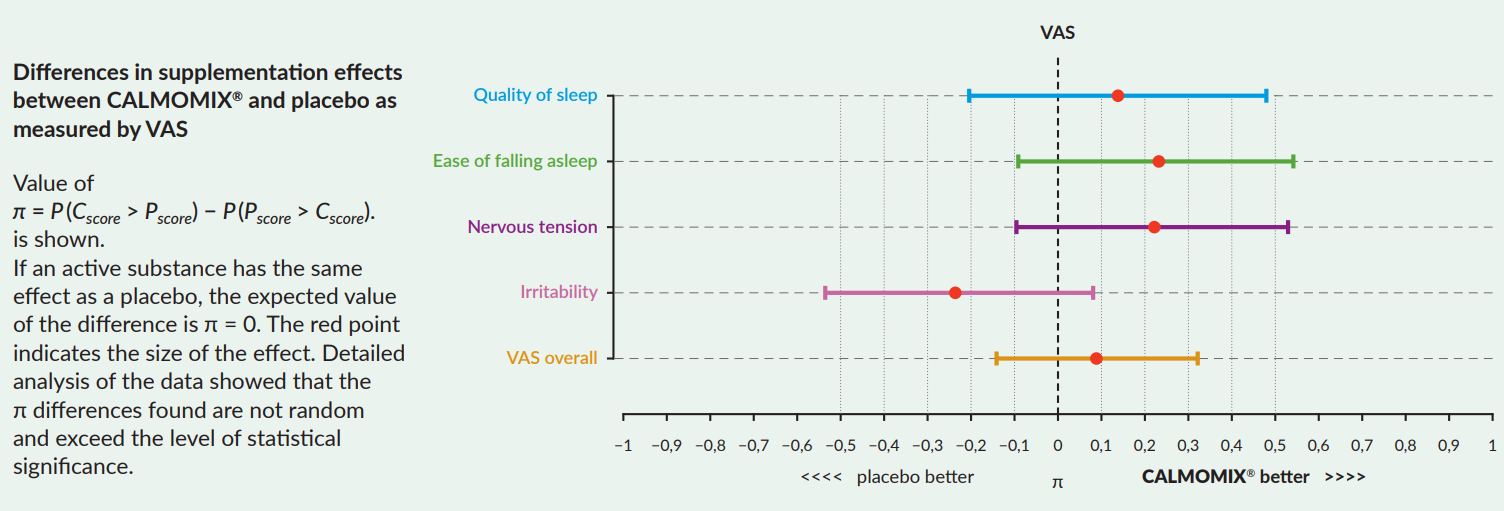
Study conclusion: the use of CALMOMIX® (a standardized complex of herbal extracts rich in CNS-affecting active substances) produced noticeable and clinically significant benefits over placebo in three of the four areas of psychological well-being examined in the VAS test.